
count: [2016-07-25] [Close]
A new class of core-shell nanoparticle catalysts with edges and vertexes covered by refractory metal oxide that preferentially segregates onto these catalyst sites has been recently developed by Dr. Jue Hu, Dr. Chengxu Zhang (Institute of Plasma Physics, CAS, China) and Prof. Radosav R. Adzic (Brookhaven National Laboratory, USA) et al., which is foreseen to have the wide application in Polymer electrolyte membrane fuel cells (PEMFCs).
PEMFCs has been recognized as one of the zero-emission power sources for the future, while the slow kinetics of the oxygen reduction reaction (ORR) is one of the key obstacles to the widespread commercialization of PEMFCs. Platinum (Pt) is the most efficient and preferred choice of electrocatalyst for the cathodic oxygen reduction. However, the high cost and low utilization efficiency of the state-of-the-art Pt catalyst are the main obstacles for its broad application in PEMFCs.
Prof. Adzic's group has been focusing on the design and synthesis of core-shell structure catalysts and has developed a class of catalysts consisting of a Pt monolayer on different substrate metals and alloy, for example, Pd, Ru, Ir, Rh, PdAu, IrNi, IrRe, and AuNiFe. However, it appears that the degradation of transition metal cores in the core-shell catalysts is the major problem under harsh fuel-cell operation conditions.
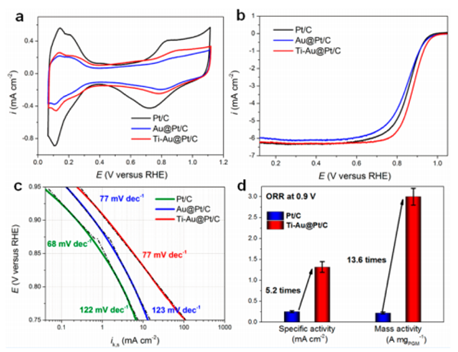
To overcome these problems, ASIPP scientists have developed a novel Au@Pt core-shell catalyst with the low-coordinated surface sites doped by titanium oxide, e.g. vertex and edges, while single atom layer Pt occupies the surface facets. The as-prepared Ti-Au@Pt/C has a more effective hydrogenation of OHad on its Pt surface than commercial Pt/C catalyst, and exhibits a 5-fold enhancement of specific activity (1.32 mA cm–2) and 13-fold enhancement of mass activity (3.0 A mg–1) relative to the commercial Pt/C catalyst.
Furthermore, in the Ti-Au@Pt/C catalysts, the highly active/distorted joint edges and corners are sealed by Ti-oxide that effectively prevent Au atoms from segregating onto the Pt surface and thus largely retained the electrochemical surface area (ECSA) and the ORR activity after 10000 potential cycles.
The related work has been published in J. Am. Chem. Soc., DOI: 10.1021/jacs.6b04999
Article links: http://pubs.acs.org/doi/abs/10.1021/jacs.6b04999
(CHENG Junli reports)
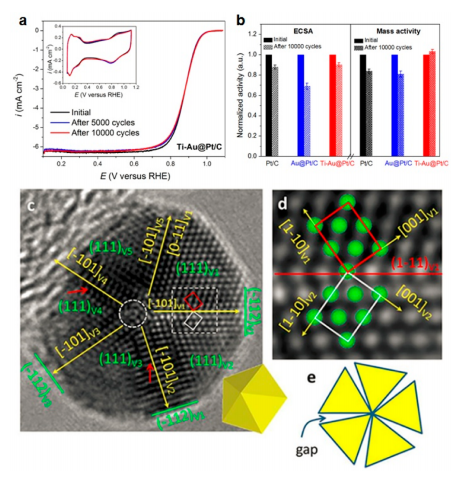
Electrochemical durability of the catalysts